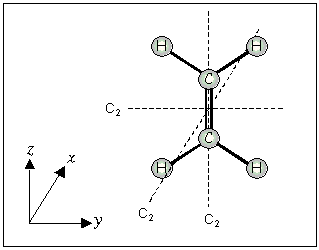
For planar molecules with the z-axis in the plane, the x-axis is perpendicular (^) to the plane.
If the z-axis is perpendicular (^) to the plane, the x-axis passes through the maximum number of atoms.
Mirror planes are related to the principal axis as follows:
| σh | — | ‘horizontal’ planes perpendicular (^) to the principal axis |
| σv | — | ‘vertical’ planes // to the principal axis |
| σd | — | ‘dihedral’ planes // to the principal axis, bisecting C2 axes |
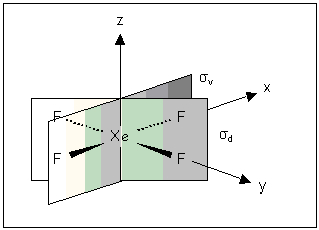
Some molecules e.g. XeF4 have two types of dihedral planes: those that bisect the F—Xe—F bonds are labelled σd; those that contain Xe—F bonds σv.
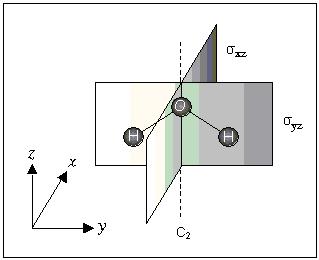
The H2O molecule has one C2 axis (z-axis) and two mirror planes designated σxz and σyz.